Super Fast Result
Only 5 minutes after Test, the result is ready
Extra Long Shelf Life
36 months Shelf Life after date of manufacture
POINT-OF-CARE TEST for COVID-19
2nd Generation Test Technology
Easy to use, producing a super fast result in only 5 minutes and with an extra long shelf life of 36 months after the date of manufacture, LUCA NK COVID-19 Ag NP LLB uses a Nasopharyngeal Swab to collect a nasopharyngeal sample for the Test
ARTG ID: 412730
- Very High Accuracy
- Very High Sensitivity
- Very High Specificity
- Test Kit size: 25 Tests Kit
- External Control Solutions included in each Test Kit
Test for COVID-19
The Test is a quick and easy to use Rapid Antigen Test (RAT) that detects the presence or absence of the SARS-CoV-2 virus (which is the virus that causes COVID-19 disease).
The Test uses an immunochromatographic assay technique to qualitatively detect the presence or absence of the nucleocapsid protein antigen of the SARS-CoV-2 virus in the sample used in the Test.
Super Fast Result
in only
5 Minutes
The Test produces a Super Fast Result in only 5 minutes using advanced 2nd Generation test technology by using the world’s first biomimetic lipid bilayer (LLB) coated diagnostic kit to provide a rapid and accurate diagnosis.
Time Saver: When carrying out multiple tests, the ability of the Test to produce a Super Fast Result in only 5 minutes can save time for the person / organisation carrying out the tests.
Extra Long Shelf Life of 36 months
after date of manufacture -
Helps reduce wastage
The Test has an Extra Long Shelf Life of 36 months after the date of manufacture, which is backed up by a scientific study carried out over that time period.
A longer Shelf Life helps you to retain your supplies of the Test for longer and helps to reduce wastage from having to throw away tests that have reached the end of their Shelf Life, which can be more of an issue with tests that have a shorter Shelf Life.
Bulk orders – Request a quote
Bulk orders of the Test are available for delivery Australia-wide.
Request a Quote now or Contact us and one of our knowledgeable and friendly staff members will assist you.
As this product is a Point-of-Care Test (not a Self-Test), it is only available for sale to certain types of persons and organisations who are authorised by the Australian Government to purchase Point-of-Care Tests.
LUCA NK COVID-19 Ag
NP LLB
A one step, immunochromatographic test for the detection of the SARS-CoV-2 antigen in human nasopharyngeal specimen
This product is not a Self-Test product and is not to be used as a Self-Test product.
This product is not available for sale to the general public.
This product is a Point-of-Care Test (also known as a “Professional Test”) which is intended to be used only by trained laboratory staff or healthcare professionals to carry out a test on another person.
This product is only available for sale to certain types of persons and organisations who are authorised by the Australian Government to purchase this type of product.
Accuracy
(Overall percent agreement)
Sensitivity (Positive percent agreement): 92.0%
Specificity (Negative percent agreement): 98.2%
Available in 25 Tests Kit size
25 Tests Kit
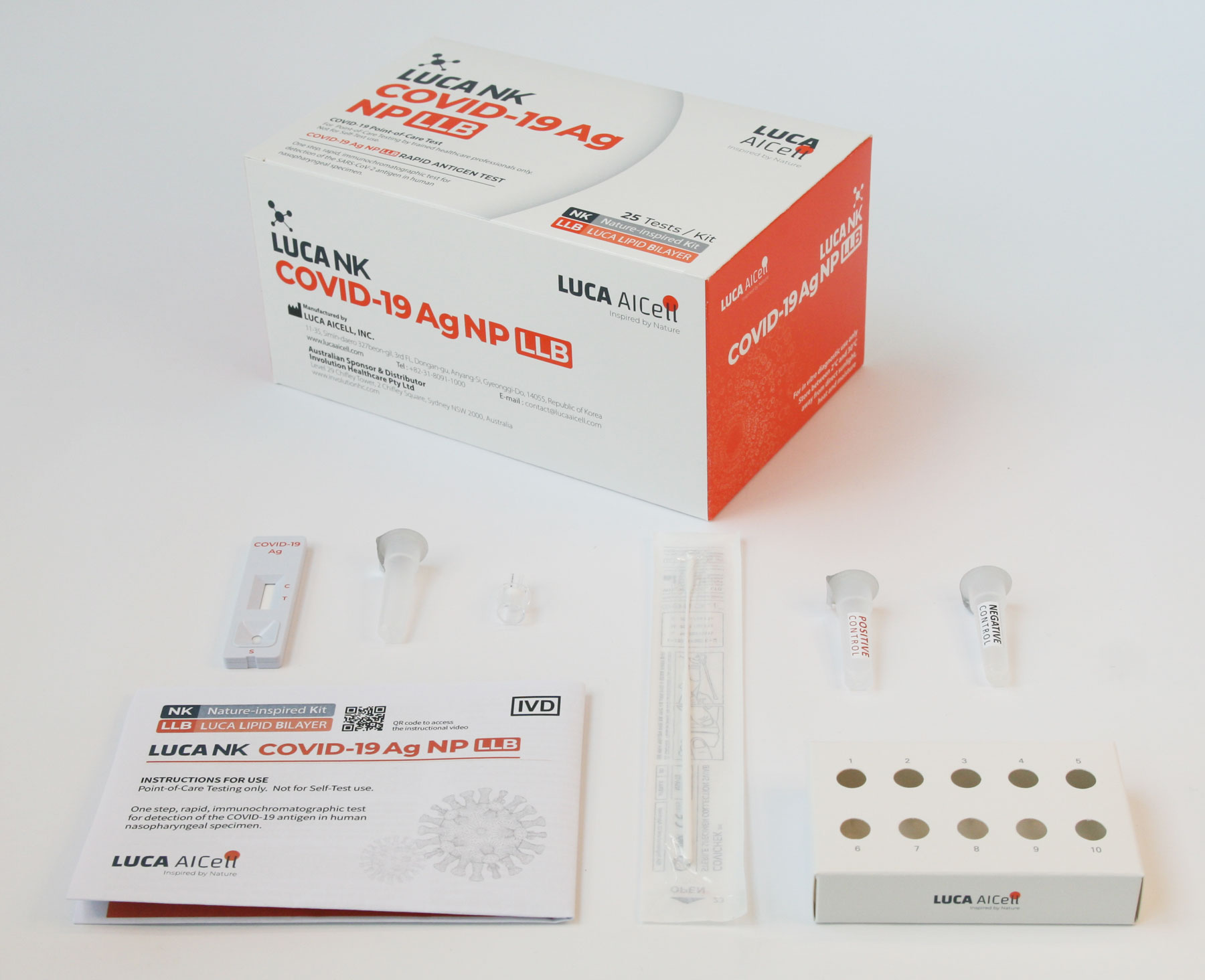
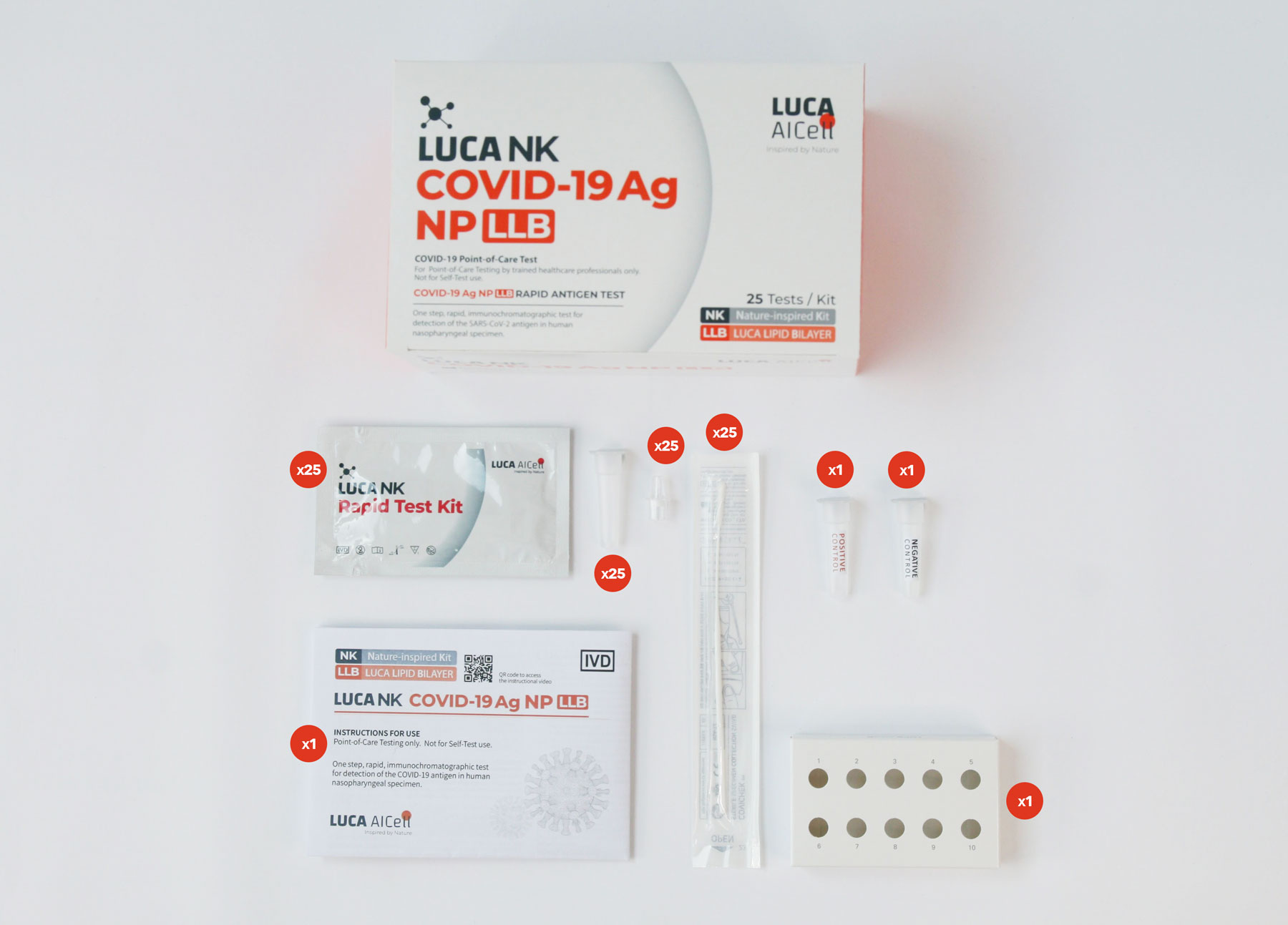
Test Kit components
The Test Kit includes all components needed to carry out a test.
There is no need for any additional components.
External Control Solutions – Included in Test Kit
Each 25 Tests Kit also includes LUCA NK CONTROL, which are control solutions (one positive and one negative), which comprise an additional External Quality Control Procedure to verify proper test performance in accordance with good laboratory practice. See further information on LUCA NK CONTROL and how to carry out the verification process in the Instructions for Use.
How to use
The Test Kit includes comprehensive Instructions for Use that give a step-by-step explanation on how to use the Test.
There is also a Video that Users can watch that explains how to use the Test
How to use Video
The How to use Video on Involution Healthcare’s YouTube page shows in video format how to administer a COVID-19 Point-of-Care test using the Test.
View the How to use Video on YouTube here:

Instructions for Use
Always follow the Instructions for Use before, during and after using the Test. The Instructions for Use are included as a paper leaflet in each Test Kit.
View and download the Instructions for Use here:
Regulatory approval in Australia
ARTG ID: 412730
The Test has obtained regulatory approval for use throughout Australia by the addition of the Test to the Australian Register of Therapeutic Goods (ARTG).
The Test has ARTG ID 412730.

TGA Q&As on COVID-19 Rapid Antigen Point-of-Care Tests
The relevant Australian Government regulatory agency, the Therapeutic Goods Administration (TGA), has published a list of Questions and Answers on COVID-19 Rapid Antigen Point-of-Care Tests.
View the TGA Q&As here:
We are waiting for you to contact us about
LUCA NK COVID-19 Ag NP LLB
High quality medical and healthcare products
Involution Healthcare are bringing next-generation pioneering technologies to Australia and New Zealand